GCSE Combined Science - Revision
Revision areas
BIOLOGY
Paper 1
Unit 1: Cell biology
- Eukaryotes and prokaryotes
- Animal and plant cells
- Cell specialisation
- Cell differentiation
- Microscopy
- Chromosomes
- Mitosis and the cell cycle
- Stem cells
- Diffusion
- Osmosis
- Active transport
Unit 2: Organisation
- Principles of organisation
- The human digestive system
- The heart and blood vessels
- Blood
- Coronary heart disease
- Health issues
- The effect of lifestyle on some non-communicable diseases
- Cancer
- Plant tissues
- Plant organ system
Unit 3: Infection and response
- Communicable (infectious) diseases
- Viral diseases
- Bacterial diseases
- Fungal diseases
- Protist diseases
- Human defence systems
- Vaccination
- Antibiotics and painkillers
- Discovery and development of drugs
Unit 4: Bioenergetics
- Photosynthetic reaction
- Rate of photosynthesis
- Uses of glucose from photosynthesis
- Aerobic and anaerobic respiration
- Response to exercise
- Metabolism
Required practical activities
- Required practical activity 1: Use a light microscope to observe, draw and label a selection of plant and animal cells.
- Required practical activity 2: Investigate the effect of a range of concentrations of salt or sugar solutions on the mass of plant tissue.
- Required practical activity 3: Use qualitative reagents to test for a range of carbohydrates, lipids and proteins.
- Required practical activity 4: Investigate the effect of pH on the rate of reaction of amylase enzyme.
- Required practical activity 5: Investigate the effect of light intensity on the rate of photosynthesis using an aquatic organism such as pondweed.
Paper 2
Unit 5: Homeostasis and response
- Homeostasis
- The human nervous system
- Human endocrine system
- Control of blood glucose concentration
- Hormones in human reproduction
- Contraception
- The use of hormones to treat infertility (HT only)
- Negative feedback (HT only)
Unit 6: Inheritance, variation and evolution
- Sexual and asexual reproduction
- Meiosis
- DNA and the genome
- Genetic inheritance
- Inherited disorders
- Sex determination
- Variation
- Evolution
- Selective breeding
- Genetic engineering
- Evidence for evolution
- Fossils
- Extinction
- Resistant bacteria
- Classification of living organisms
Unit 7: Ecology
- Communities
- Abiotic factors
- Biotic factors
- Adaptations
- Levels of organisation
- How materials are cycled
- Biodiversity
- Waste management
- Land use
- Deforestation
- Global warming
- Maintaining biodiversity
Required practical activities
- Required practical activity 6: Plan and carry out an investigation into the effect of a factor on human reaction time.
- Required practical activity 7: Measure the population size of a common species in a habitat. Use sampling techniques to investigate the effect of a factor on the distribution of this species.
CHEMISTRY
Paper 1
Unit 8: Atomic structure and the periodic table
- Atoms, elements and compounds
- Mixtures
- The development of the model of the atom
- Relative electrical charges of subatomic particles
- Size and mass of atoms
- Relative atomic mass
- Electronic structure
- The periodic table
- Development of the periodic table
- Metals and non-metals
- Group 0
- Group 1
- Group 7
Unit 9: Bonding, structure, and the properties of matter
- Chemical bonds
- Ionic bonding
- Ionic compounds
- Covalent bonding
- Metallic bonding
- The three states of matter
- State symbols
- Properties of ionic compounds
- Properties of small molecules
- Polymers
- Giant covalent structures
- Properties of metals and alloys
- Metals as conductors
- Diamond
- Graphene and fullerenes
Unit 10: Quantitative chemistry
- Conservation of mass and balanced chemical equations
- Relative formula mass
- Mass changes when a reactant or product is a gas
- Chemical measurements
- Moles (HT only)
- Amounts of substances in equations (HT only)
- Using moles to balance equations (HT only)
- Limiting reactants (HT only)
- Concentration of solutions
Unit 11: Chemical changes
- Metal oxides
- The reactivity series
- Extraction of metals and reduction
- Oxidation and reduction in terms of electrons (HT only)
- Reactions of acids with metals
- Neutralisation of acids and salt production
- Soluble salts
- The pH scale and neutralisation
- Strong and weak acids (HT only)
- The process of electrolysis
- Electrolysis of molten ionic compounds
- Using electrolysis to extract metals
- Electrolysis of aqueous solutions
- Representation of reactions at electrodes as half equations (HT only)
Unit 12: Energy changes
- Energy transfer during exothermic and endothermic reactions
- Reaction profiles
- The energy change of reactions (HT only)
Required practical activities
- Required practical activity 8: Preparation of a pure, dry sample of a soluble salt from an insoluble oxide or carbonate.
- Required practical activity 9: Investigate what happens when aqueous solutions are electrolysed.
- Required practical activity 10: Investigate the variables that affect temperature changes in reacting solutions.
Paper 2
Unit 13: The rate and extent of chemical change
- Calculating rates of reactions
- Factors which affect the rates of chemical reactions
- Collision theory and activation energy
- Catalysts
- Reversible reactions
- Energy changes and reversible reactions
- Equilibrium
- The effect of changing conditions on equilibrium (HT only)
- The effect of changing concentration (HT only)
- The effect of temperature changes on equilibrium (HT only)
- The effect of pressure changes on equilibrium (HT only
Unit 14: Organic chemistry
- Crude oil, hydrocarbons and alkanes
- Fractional distillation and petrochemicals
- Properties of hydrocarbons
- Cracking and alkenes
Unit 15: Chemical analysis
- Pure substances
- Formulations
- Chromatography
- Identification of common gases
Unit 16: Chemistry of the atmosphere
- The proportions of different gases in the atmosphere
- The Earth's early atmosphere
- How oxygen increased
- How carbon dioxide decreased
- Greenhouse gases
- Human activities which contribute to an increase in greenhouse gases in the atmosphere
- Global climate change
- The carbon footprint and its reduction
- Atmospheric pollutants from fuels
- Properties and effects of atmospheric pollutants
Unit 17: Using resources
- Using the Earth's resources and sustainable development
- Potable water
- Waste water treatment
- Alternative methods of extracting metals (HT only)
- Life cycle assessment
- Ways of reducing the use of resources
Required practical activities
- Required practical activity 11: Investigate how changes in concentration affect the rates of reactions.
- Required practical activity 12: Investigate how paper chromatography can be used to separate and tell the difference between coloured substances.
- Required practical activity 13: Analysis and purification of water samples from different sources, including pH, dissolved solids and distillation.
PHYSICS
Paper 1
Unit 18: Energy
- Energy stores and systems
- Changes in energy
- Energy changes in systems
- Power
- Energy transfers in a system
- Efficiency
- National and global energy resources
Unit 19: Electricity
- Standard circuit diagram symbols
- Electrical charge and current
- Current, resistance and potential difference
- Resistors
- Series and parallel circuits
- Direct and alternating potential difference
- Mains electricity
- Power
- Energy transfers in everyday appliances
- The National Grid
Unit 20: Particle model of matter
- Density of materials
- Changes of state
- Internal energy
- Temperature changes in a system and specific heat capacity
- Changes of heat and specific latent heat
- Particle motion in gases
Unit 21: Atomic structure
- The structure of an atom
- Mass number, atomic number and isotopes
- The development of the model of the atom
- Radioactive decay and nuclear radiation
- Nuclear equations
- Half-lives and the random nature of radioactive decay Content K
- Radioactive contamination
Required practical activities
- Required practical activity 14: An investigation to determine the specific heat capacity of materials.
- Required practical activity 15: Use circuit diagrams to set up and check appropriate circuits to investigate the factors affecting the resistance of electrical circuits.
- Required practical activity 16: Use circuit diagrams to construct appropriate circuits to investigate the I–V characteristics of a variety of circuit elements.
- Required practical activity 17: Use appropriate apparatus to make and record the measurements needed to determine the densities of regular and irregular solid objects and liquids.
Paper 2
Unit 22: Forces
- Scalar and vector quantities
- Contact and non-contact forces
- Gravity
- Resultant forces
- Work done and energy transfer
- Forces and elasticity
- Describing motion along a line
- Speed
- Velocity
- The distance–time relationship
- Acceleration
- Forces, accelerations and Newton's Laws of motion
- Stopping distance
- Reaction time
- Factors affecting braking distance
- Momentum (HT only)
Unit 23: Waves
- Transverse and longitudinal waves
- Properties of waves
- Types of electromagnetic waves
- Properties of electromagnetic waves
- Uses and applications of electromagnetic waves
Unit 24: Magnetism and electromagnetism
- Poles of a magnet
- Magnetic fields
- Electromagnetism
- Fleming's left-hand rule (HT only)
- Electric motors (HT only)
Required practical activities
- Required practical activity 18: Investigate the relationship between force and extension for a spring.
- Required practical activity 19: Investigate the effect of varying the force on the acceleration of an object of constant mass, and the effect of varying the mass of an object on the acceleration produced by a constant force.
- Required practical activity 20: To measure the frequency, wavelength and speed of waves in a ripple tank and waves in a solid.
- Required practical activity 21: Investigate how the amount of infrared radiation absorbed or radiated by a surface depends on the nature of that surface.
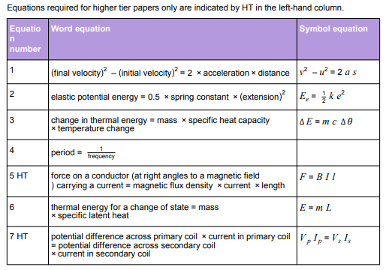
Physics equations students are required to learn
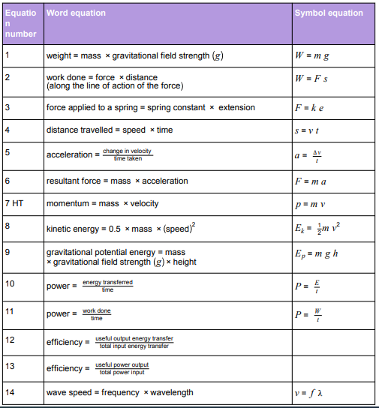
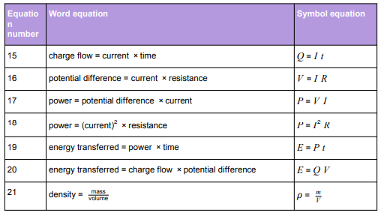
Physics equations students will be provided with in the exam